Jan 8, 2013 — Metal organic source cylinders (bubblers) are stored in the constant temperature water baths. Hazardous materials that will be used (see
21 pages
194 KB – 21 Pages
PAGE – 1 ============
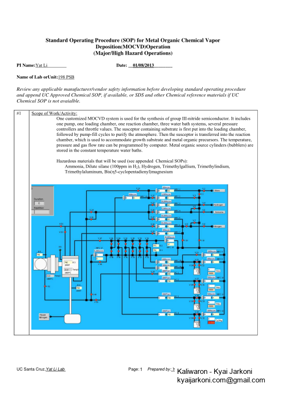
UC Santa Cruz, Yat Li Lab Page: Prepared by: Yat Li and Yichuan Ling 1Standard Operating Procedure (SOP) for Metal Organic Chemical Vapor Deposition (MOCVD)Operation (Major/High Hazard Operations) PI Name: Yat Li Date: __01/08/2013 ________ Name of Lab orUnit: 198 PSB Review any applicable manufacturer/vendor safety information before developing standard operating procedure and append UC Approved Chemical SOP, if available, or SDS and other Chemical reference materials if UC Chemical SOP is not avaialble. #1 Scope of Work/Activity: One customized MOCVD system is used for the synthesis of group III-nitride semiconductor. It includes one pump, one loading chamber, one reaction chamber, three water bath systems, several pressure controllers and throttle values. The susceptor containing substrate is first put into the loading chamber, followed by pump-fill cycles to purify the atmosphere. Then the susceptor is transferred into the reaction chamber, which is used to accommodate growth substrate and metal organic precursors. The temperature, pressure and gas flow rate can be programmed by computer. Metal organic source cylinders (bubblers) are stored in the constant temperature water baths. Hazardous materials that will be used (see appended Chemical SOPs): Ammonia, Dilute silane (100ppm in H 2), Hydrogen, Trimethylgallium, Trimethylindium, Trimethylaluminum, Bis( 5-cyclopentadienyl)magnesium
PAGE – 2 ============
UC Santa Cruz, Yat Li Lab Page: Prepared by: Yat Li and Yichuan Ling 2#2 Specific Safety and Environmental Hazards: State the specific hazard and consequences if procedure not followed to person, environment, or property. Release of process gases and/or metal organic precursors into hood enclosure and/or lab can result in fire and chemical exposure. User contact with hot surfaces can result in burns.
PAGE – 3 ============
UC Santa Cruz, Yat Li Lab Page: Prepared by: Yat Li and Yichuan Ling 3#3 Describe in detail how the hazards will be controlled. Hazard 1: There is a chance of gas leakage into fume hood if the user fails to get a good seal of the loading chamber. The process gases that may be involved in the reaction include ammonia, diluted silane (100ppm in H 2), and hydrogen. User: Make sure the MOCVD system is leak tight during the pump-fill cycles before transferring the susceptor into the reaction chamber and starting the reaction program. The fume hood door should be closed all the time except while loading the sample and transferring the susceptor. Hazard 2: There is a chance of metal organic precursor leakage from bubbler if the bubbler is not installed properly and leak checked. User: Follow the bubbler change-out SOP and conduct leak check. Hazard 3: High temperature reaction (up to 1300 oC). User: DO NOT change the position of the thermocouples Double check the temperature setting before the experiment, and monitor the temperature during the reaction. DO NOT take out the susceptor before it is cooled down (below 50 oC) No flammable solvent in the hood Hazard 4: Vacuum system User: Open the pump and subsidiary nitrogen gas flow. DO NOT pressurize the system. The reaction pressure should not exceed atmospheric pressure Double check the pressure controller setting before the experiment Monitor the gas flow rate and pressure of the reactor during the reaction Hazard 5: Water cooling system. User: Open the four cooling water values before starting the pump. The whole MOCVD will automatically be shut down when the pump starts working without cooling water for 3 minutes. If this happens, turn off the pump and check the cooling water system. Hazard 6: Chemicals User: Trimethylgallium, Trimethylindium, Trimethylaluminum, Bis( 5-cyclopentadienyl)magnesium are pyrophoric and water reactive chemicals. These compounds ignite in air and may react explosively with water. They are always used in a closed, purged system. Ammonia is corrosive and flammable. It is a colorless highly irritating gas with a sharp suffocating odor. The ammonia is stored in a ventilated gas cabinet and always used in a closed, purged system. Silane (100 ppm in hydrogen). Silane is a pyrophoric (at concentrations > 4.5%) and toxic gas. At 100 ppm, the silane concentration is below its lower flammability limit, however, hydrogen is a flammable gas. The silane/hydrogen mixture is stored in a ventilated gas cabinet and always used in a closed, purged system. Engineering Controls The MOCVD system are installed in a walk-in fumehood. The fumehood door is closed during the experiment except loading sample and transferring susceptor. Interlocks are provided for overtemperature protection and automatic gas shut off in case of leak detection and/or excess flow. Emergency shutdown procedures. Press the red fiEmergency Stopfl button near the door to shut down all gas supply and shut off power to the MOCVD system. #4 Designated Area: Indicate the designated area for performing this process in the laboratory. MOCVD systems are installed in a walk-in fumehood. The fumehood door is closed during the experiment except while loading sample and transferring susceptor.
PAGE – 4 ============
UC Santa Cruz, Yat Li Lab Page: Prepared by: Yat Li and Yichuan Ling 4#5 Personal Protective Equipment (PPE): User should wear gloves, safety glasses, and flame resistant lab coat. User should not wear contact lenses. For precursor bubbler change out: wear safety glasses, face shield, Nomex lab coat, Nomex pilot™s gloves. (Refer to bubbler change-out SOP). #6 Important Steps to Follow: List the specific sequence staff should follow to avoid hazard. 1. Connect the power of the pump. Open the four cooling water values. (Cooling water returns first) 2. Load susceptor with substrate into the loading chamber and close the chamber door firmly. 3. Turn on the nitrogen connected to the pump. Make sure the fiCooling water failurefl light is off, and then press the fiONfl button to start the pump. 4. Use the computer to start fiBack Fill Load Chamberfl program to do the pump-fill cycles. 5. When the pump-fill cycles are done, open the gate between loading chamber and reaction chamber and send susceptor into reaction chamber. 6. Raise the heater until it touch the bottom of the susceptor. 7. In computer, choose the correct reaction program via fiLoad Recipefl. 8. Switch the control value (below the reaction chamber) from fiholdfl to fiNormalfl. 9. Turn on the power supply of the heater, then click fiRun Recipefl in the computer to start reaction. 10. When the reaction is done, switch the control value (below the reaction chamber) from fiNormalfl to fiholdfl, turn off the pump as well as the nitrogen gas cylinder. 11. When the temperature of the reaction is back to room temperature, start fiVent both Chamberfl program in computer to adjust the pressure. Then open the gate to transfer the susceptor back to the loading chamber. Close the gate. 12. Make sure the reaction is well sealed. Then open loading chamber to take the susceptor out. #7 Emergency First Aid Procedures: If inhaled If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician. In case of skin contact Take off contaminated clothing and shoes immediately. Wash off with soap and plenty of water. Consult a physician (bring Material Safety Data Sheet with you). In case of eye contact Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician. Continue rinsing eyes during transport to hospital. If swallowed Do NOT induce vomiting. Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician. #8 Training & Competency Requirements: Prior to entering and working in the laboratory, you must have completed the EH&S fiIntroduction to Laboratory Safetyfl class and the Lab-Specific Training Checklist. Review SOP with LSR and PI New user can be get trained by any experienced user, but should be qualified by Yat Li or Yichuan Ling. #9 Identify waste stream and disposition of unused stock of chemicals Unreacted precursors in bubblers are to be shipped back to supplier.
PAGE – 5 ============
UC Santa Cruz, Yat Li Lab Page: Prepared by: Yat Li and Yichuan Ling 5#10 Decontamination and spill clean-up procedures. Do not attempt to clean up any spill or release for which you are not fully trained and equipped. Contact 911 and ask for EH&S assistance for spill cleanup. In the event of a spill or gas release: a. Alert people in the laboratory to evacuate. b. Press the red fiEmergency Stopfl button by the door to shut down the MOCVD and stop gas flow. c. Close doors to affected area. d. Call for Emergency Response: 911 e. Post with danger signs and have person knowledgeable of incident and laboratory assist emergency personnel #11 Laboratory Emergency Response Equipment: All research personnel must know location of nearest fire alarm pull station and emergency shower/eyewash. a. Note location and use of any emergency response equipment specific to process (e.g., Calgonate gel, Class D fire extinguisher) Item Location Eyewash/Safety Shower Near the door Chemical Spill Kit N/A Fire Extinguisher Outside the door Telephone Student office First Aid Kit On the shelf Fire Alarm Manual Pull Station Outside the door As the Principal Investigator, it is your responsibility to ensure that all individuals listed in this protocol are taught the correct procedures for the safe handling of hazardous materials involved in this study. It is also your responsibility to assure that y our personnel complete Laboratory Safety Training and other applicable safety training courses. I have read, asked questions, and understand the hazards of and safe working procedures for the activity/materials described herein. 1/9/2013 PI Signature: DATE
PAGE – 6 ============
UC Santa Cruz, Yat Li Lab Page: Prepared by: Yat Li and Yichuan Ling 6 Chemical Hazards and Controls Ammonia This is a Chemical Hazard and Control template and is no t complete until: 1) Chemical specific information is entered into the boxes below 2) Itis appended to the protocol/procedural SOP and 3) Complete SOP has been signed and dated by the PI and relevant lab personnel. Department: Chemistry Date SOP was written: 01/08/13 Date SOP was approved by PI/lab supervisor: Principal Investigator: Yat Li Internal Lab Safety Coordinator/Lab Manager: Tianyu Liu Lab Phone: Office Phone: (831) 459-1952 Emergency Contact: Yat Li (Name and Phone Number) Location(s) covered by this SOP: PSB 198 (Building/Room Number) Physical & Chemical Properties /Definition of Chemical Group CAS#: 7664-41-7 Class: Molecular Formula: H 3N Form (physical state): compressed gas, strong odor Color: colorless Boiling point: -33 oC at 1 atm pressure. Melting point: -78 oC Density = 0.590 g/mL Potential Hazards/Toxicity Ammonia is both caustic and hazardous. Ammonia is a colorless highly irritating gas with a sharp suffocating odor. It dissolves easily in water to form ammonium hydroxide solution which can cause irritation and burns. Because NH 3 boils at 33.34 °C ( 28.012 °F) at a pressure of 1 atmosphere, the liquid must be stored under high pressure or at low temperature. It is usually shipped as a compressed liquid in steel cylinders. Ammonia is not highly flammable at very low or very high concentrations; CAUTION avoid a moderate gas release in a closed environment (room) with an ignition source (light switch) and oxygen present [Lower explosion limit 15 %(V) with Upper explosion limit 25 %(V)]. Containers of ammonia may explode when exposed to high heat.
PAGE – 8 ============
UC Santa Cruz, Yat Li Lab Page: Prepared by: Yat Li and Yichuan Ling 8Engineering Controls Cylinder is stored/used in a certified ventilated gas cabinet and directly plumbed to the MOCVD located in a walk-in fume hood. System is equipped with emergency stop switch to shut down gas flow. First Aid Procedures If inhaled If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician. In case of skin contact Take off contaminated clothing and shoes immediately. Wash off with soap and plenty of water. Take victim immediately to hospital. Consult a physician (bring Material Safety Data Sheet with you). In case of eye contact Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician. Continue rinsing eyes during transport to hospital. If swallowed Do NOT induce vomiting. Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician. Special Handling and Storage Requirements Precautions for safe handling Handle this material only in sealed, purged systems. Handle sealed gas cylinders in accordance with CGA P-1, Safe Handling of Compressed Gases in Containers. Keep away from sources of ignition. Take measures to prevent the buildup of electrostatic charge. Conditions for safe storage Keep cylinder in ventilated gas cabinet. Contents under pressure. Incompatible with the Following Materials Materials to avoid Oxidizing agents, Iron, Zinc, Copper, Silver/silver oxides, Cadmium/cadmium oxides, Alcohols, acids, Halogens, Aldehydes Spill and Accident Procedure Release ΠDial 911 and ask for EH&S assistance. Alert people in the laboratory to evacuate. Press the red fiEmergency Stopfl button by the door to shut down the MOCVD and stop gas flow. Close doors to affected area. Post with danger signs and have person knowledgeable of incident and laboratory assist emergency responders Chemical Spill Dial 911 Spill ΠDial 911 and ask for EH&S assistance or call EH&S directly x459-2553. Chemical Spill on Body or Clothes ΠRemove clothing and rinse body thoroughly in emergency shower for at least 15 minutes. Seek medical attention if needed. Notify supervisor and EH&S via 911 immediately. Chemical Splash Into Eyes ΠImmediately rinse eyeball and inner surface of eyelid with water from the emergency eyewash station for 15 minutes by forcibly holding the eye open. Seek medical attention. Notify supervisor and EH&S via 911 immediately.
PAGE – 9 ============
UC Santa Cruz, Yat Li Lab Page: Prepared by: Yat Li and Yichuan Ling 9Mucous Membrane Exposure : Flush the affected area for 15 minutes using an eyewash station. Needlestick/Puncture Injuries ΠWash the affected area with soap and warm water for 15 minutes. For employees, follow the instructions at the Risk Services website: http://risk.ucsc.edu/workerscomp/injuryreportinghowto.html Medical Emergency Dial 911 Life Threatening Emergency, After Hours, Weekends And Holidays ΠDial 911 Non-Life Threatening Emergency ΠFor employees, follow the instructions at the Risk Services website: http://risk.ucsc.edu/workerscomp/injuryreportinghowto.html Note : All serious injuries must be reported to EH&S as soon as possible. Decontamination/Waste Disposal Procedure Cylinders that contained ammonia must be returned to the supplier. Waste Procedures General hazardous waste management guidelines: http://ehs.ucsc.edu/programs/waste-management/index.html Contact EH&S at x9-3086 for questions Safety Data Sheet (SDS) Location Online SDSs can be accessed at: http://www.ucmsds.com/?X . NOTE Any deviation from this Procedural/Chemical Handling SOP requires approval from PI.
PAGE – 10 ============
UC Santa Cruz, Yat Li Lab Page: Prepared by: Yat Li and Yichuan Ling 10Chemical Hazards and Controls Silane (100 ppm in Hydrogen) This is a Chemical Hazard and Control template and is no t complete until: 1) Chemical specific information is entered into the boxes below 2) Itis appended to the protocol/procedural SOP and 3) Complete SOP has been signed and dated by the PI and relevant lab personnel. Purpose Silane is purchased as a 100 ppm mixture in hydrogen for use in the MOCVD system in the lab. The information below, for pure silane gas, is provided for reference. See the Ammonia SOP above for Engineering Controls, First Aid, and Chemical Release and Accident Procedures. Physical & Chemical Properties /Definition of Chemical Group CAS#: 7803-62-5 Class: Pyrophoric and highly flammable gas Molecular Formula: SiH4 Molecular Weight: 32.12 g/mol Form (physical state): gas Color: colorless Melting Point:-185 °C Boiling point:-112.15 °C Lower Flammability Limit (in air) = 1.4% Upper Flammability Limit (in air) = 96.0% May undergo bulk autoignition at > 4.5% Potential Hazards/Toxicity Pyrophoric, flammable, high-pressure gas. Can ignite on contact with air. May form explosive mixtures with air. Does not need a source of ignition. Respiratory irritant. May cause respiratory system damage. Self-contained breathing apparatus and protective clothing may be required by rescue workers. Under ambient conditions, this colorless gas has a choking odor. Cal-OSHA PEL = 5 ppm.
PAGE – 11 ============
UC Santa Cruz, Yat Li Lab Page: Prepared by: Yat Li and Yichuan Ling 11Chemical Hazards and Controls Hydrogen This is a Chemical Hazard and Control template and is not complete until: 1) Chemical specific information is entered into the boxes below 2) Itis appended to the protocol/procedural SOP and 3) Complete SOP has been signed and dated by the PI and relevant lab personnel. Purpose Hydrogen (H2) is a highly flammable gas. Hydrogen gas forms explosive mixtures with air if it is 4Œ74% concentrated and forms explosive mixtures with chlorine if it is 5Œ95% concentrated. The mixtures spontaneously explode by spark, heat or sunlight. Auto-ignition temperature of Hydrogen: The temperature of spontaneous ignition in air, is 500 °C (932 °F). The detection of a burning hydrogen leak may require a flame detector; such leaks can be very dangerous. Hydrogen reacts with every oxidizing element. Hydrogen poses a number of hazards to human safety, from potential detonations and fires when mixed with air to being an asphyxiant in its pure, oxygen-free form. Hydrogen dissolves in many metals. In addition to leaking out, may have adverse effects on metals, such as hydrogen embrittlement, leading to cracks and explosions. Hydrogen gas leaking into external air may spontaneously ignite. Moreover, hydrogen fire, while being extremely hot, is almost invisible, and thus can lead to accidental burns. Even interpreting the hydrogen data (including safety data) is confounded by a number of phenomena. Hydrogen detonation parameters such as critical detonation pressure and temperature, strongly depend on the container geometry. If not handled and stored properly, Hydrogen gas can pose a serious threat to the health and safety of laboratory personnel & emergency responders and also to the property. This SOP helps to understand how to properly store & handle hydrogen. Physical & Chemical Properties /Definition of Chemical Group CAS#: 1333-74-0 Class: Highly flammable gas Molecular Formula: H 2 Form (physical state): compressed gas Color: colorless Boiling point: -252.87 °C Melting point: -259.14 °C Density = 0.08988 g/L Potential Hazards/Toxicity Hydrogen (H2) is a highly flammable gas. Hydrogen gas (dihydrogen or molecular hydrogen) is highly flammable and will burn in air at a very wide range of concentrations between 4% and 75% by volume. Personal Protective Equipment (PPE) Respiratory Protection Respirators should be used only under any of the following circumstances: As a last line of defense (i.e., after engineering and administrative controls have been exhausted).
194 KB – 21 Pages